Intro To Intermolecular Forces Pogil Answers | Rank the following from weakest intermolecular forces to strongest. Taken from the answer key learn with flashcards, games and more — for free. Intramolecular forces occur within/inside molecules, while intermolecular forces occur between molecules. Without intermolecular forces holding molecules together we would not exist. Baxley intermolecular forces worksheet answers are on page 3 & 4.
Transcribed image text from this question. Do not type units into the answer boxes, type only the numeric values. Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions ). How much heat is required to melt 42.7 g of diethyl ether? H2se h2s h2po h2te chem128.
Different types of intermolecular forces (forces between molecules). This one was quite a bit. Baxley intermolecular forces worksheet answers are on page 3 & 4. Intermolecular forces (imf) are the forces which cause real gases to deviate from ideal gas behavior. That is the force responsible for holding water molecules together, and. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them. Which of these is not an intermolecular force? Page 1 of 7 name date block pogil: Taken from the answer key learn with flashcards, games and more — for free. 1 name date block pogil: This only occurs when there is a is difluormethane polar or non polar? Intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules.
1.what specific molecule is represented inside. In fact, groups of molecules stick. What are these intermolecular forces? The strongest intermolecular force is hydrogen bonding which is the force of attractiong between a h atom which is covalently bonded to the lone pair of one of the strongest intermolecular forces (i.e. Forces which exist within same molecule or a polyatomic ion ,affect the chemical properties of the substance.
London dispersion forces are attractive forces that exist between all atoms and molecules. A chapter eleven sexual problem. Without intermolecular forces holding molecules together we would not exist. Do the problems on your own before looking at the answers. Thank you for submitting your answer. What is an intermolecular force? Hydrogen bonding van der waals are the weakest van der waal forces exist between all molecules in the universe. 1.what specific molecule is represented inside. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Answer the following to the best of your ability. The more intermolecular forces, the higher the boiling point. Do not type units into the answer boxes, type only the numeric values.
This one was quite a bit. Rank the following from weakest intermolecular forces to strongest. Intramolecular forces (bonding forces) exist within molecules and influence the chemical properties. Ø only polar species are involved in intermolecular attractive forces should be drawn between two ends of opposite charge. Taken from the answer key learn with flashcards, games and more — for free.
Page 1 of 7 name date block pogil: Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them. Which of these is not an intermolecular force? Rank the following from weakest intermolecular forces to strongest. Transcribed image text from this question. Do not use commas or scientific notation when entering large numbers. Ø only polar species are involved in intermolecular attractive forces should be drawn between two ends of opposite charge. They are also responsible for the formation of the condensed phases, solids and liquids. Intermolecular forces (imf) (or secondary forces) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between atoms and other types of. Greater the intermolecular forces, higher is the boiling point. Intramolecular forces occur within/inside molecules, while intermolecular forces occur between molecules. Do the problems on your own before looking at the answers. These forces are weak compared to the intramolecular forces, such as the covalent or ionic bonds between atoms in a molecule.
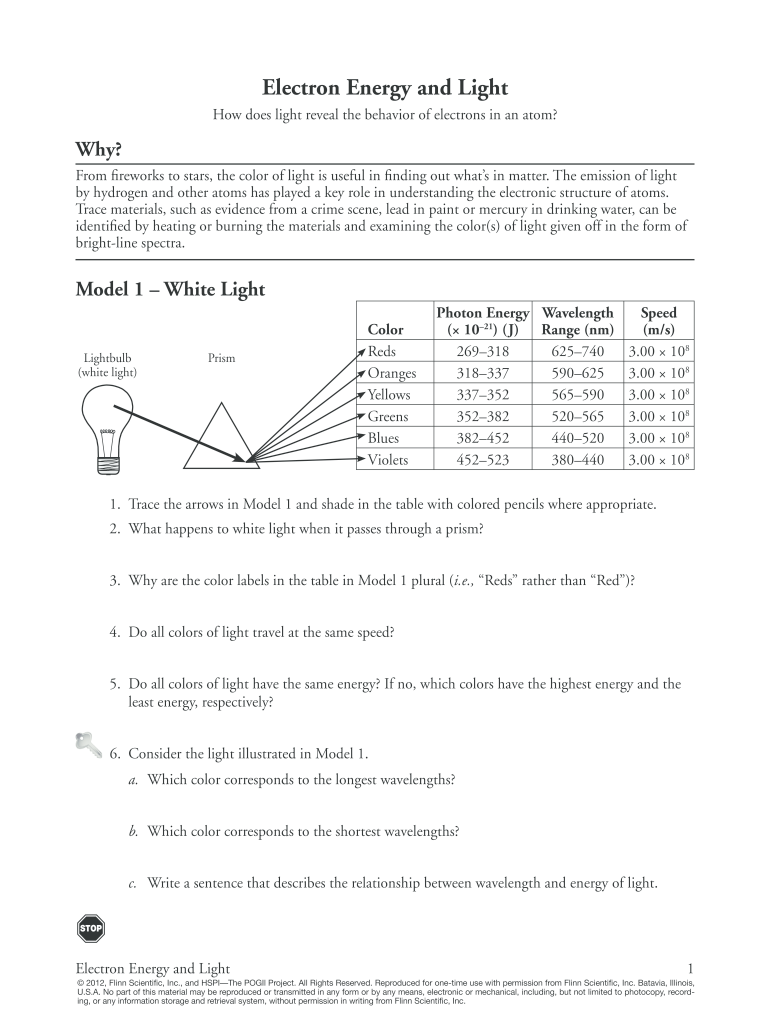
Intro To Intermolecular Forces Pogil Answers: The intermolecular forces arises due to following interactions:
EmoticonEmoticon